
Goals of HAE treatment and available treatment options
Ultimate goals of treatment
The updated 2021 World Allergy Organization/European Academy of Allergology and Clinical Immunology guideline for the management of HAE outlined the ultimate goals of treatment:1

Complete control of HAE essentially translates to patients living their lives free from any attacks. In cases where complete control of the disease cannot be achieved, the goal of treatment should be to reduce the number of attacks as much as possible and improve the patient’s quality of life.1
Treatment types
Currently, there are two treatment modalities for patients with HAE: on-demand (or acute) treatment for when HAE attacks occur, and prophylactic therapy to prevent attacks from occurring.1
On-demand treatment
- Administered in the event of an HAE attack
- Shortens the time to attack resolution

Prophylactic treatment
- Regular administration
- Reduces attack frequency/prevents attacks from occurring
On-demand therapy for HAE
On-demand treatments for HAE aim to prevent the progression of angioedema. Patients should be informed that medication is more effective if it is taken as early as possible in the event of an HAE attack, so that symptoms resolve as quickly as possible. Patients can also be trained to self-administer, which facilitates early treatment.1 Even if a patient is on long-term prophylaxis (LTP), it is recommended that:
WAO/EAACI guideline 20211
Preventing HAE attacks with LTP
WAO/EAACI guidelines state that LTP is currently the only way to achieve the goals of treatment1
LTP medication can be given regularly to prevent HAE attacks. Studies show that LTP significantly reduces the frequency and severity of HAE attacks.3-5 Currently, treatment is possible using SC, IV or oral medications, which patients or their relatives can learn to administer under certain conditions. All patients should be considered for long-term prophylaxis (LTP) as it is the only therapy with which we could reach the goals of treatment: total control of the disease and normalisation of the patient’s life.
WAO/EAACI guideline 20211
First-line long-term prophylaxis treatment options
WAO/EAACI guideline 20211
Self-administration
Guidelines recommend that patients learn to self-administer their medication at home or on the go if they are eligible1. This means they can act quickly in the event of an acute attack and have more freedom in their everyday life.
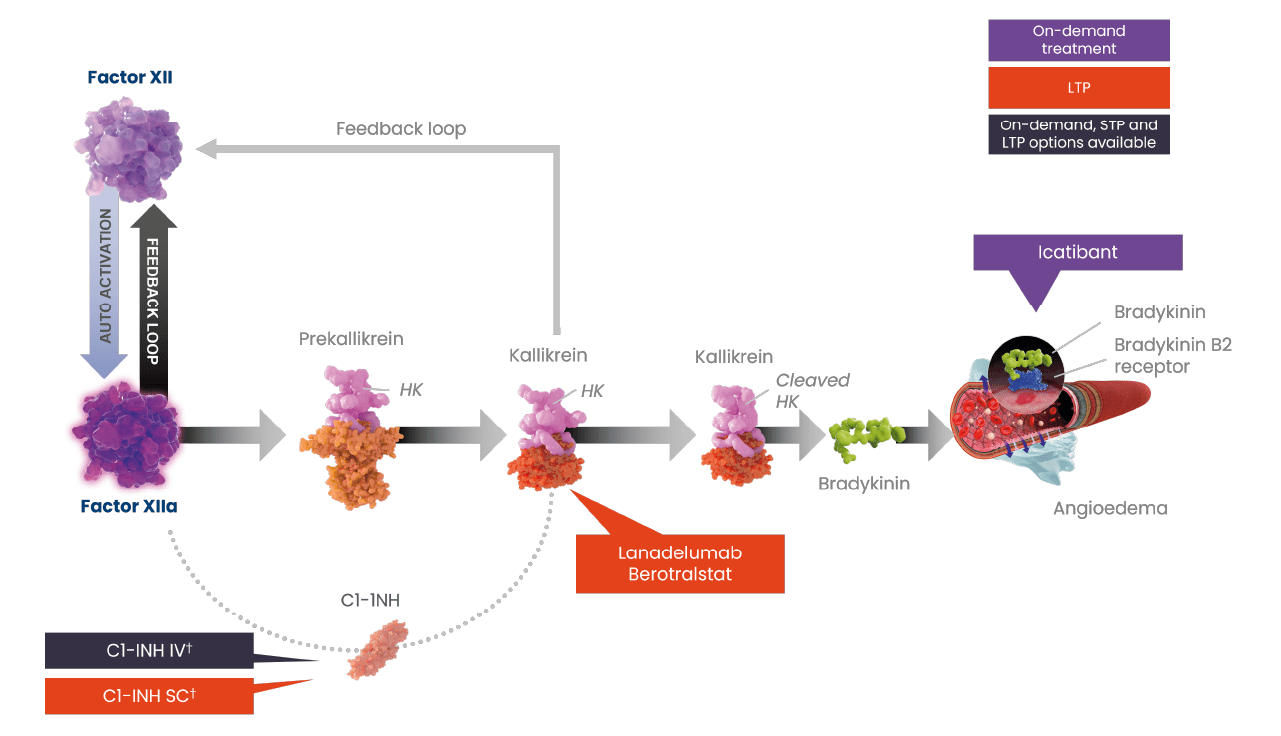
†Plasma-derived C1-INH therapy is available on-demand and for short- and long-term prophylaxis10,11; recombinant therapy is available for on-demand treatment of HAE attacks.
C1INH, C1-esterase inhibitor; FXII, coagulation factor XII; FXIIa, activated factor XII; HAE, hereditary angioedema; IV, intravenous; LTP, long-term prophylaxis; SC, subcutaneous; STP, short-term prophylaxis.
Maurer M et al. Allergy 2022;77:1961–1990; Busse P et al. J Allergy Clin Immunol Pract 2021;9:132–150.e3; Lera AL. Balkan Med J 2021;38:82–88; Kaplan AP. Blood 2022;139:2732–2733; Reshef A et al. J Allergy Clin Immunol 2024:S0091-6749(24)00407-X.
Shared decision-making:13
How can we assess whether the goals of treatment are being met?
Even if your patient is on LTP, is important to regularly monitor their disease activity, impact and control. You should ensure that, in addition to disease activity, the patient’s QoL is assessed.1 Even patients with low attack rates can have impaired QoL, which could be linked to the unpredictability of the disease and the impact this has on their lives, e.g., fear of attacks and avoiding activities that might trigger attacks.1
The WAO/EAACI guideline states that “the aims of effective treatment, i.e., the absence of attacks, normalisation of quality of life and complete control, are best achieved when assessed by appropriate tools.”1
Validated patient-reported outcome measures (PROMs) available for use in HAE can measure disease activity, disease impact on QoL, and disease control:1

As part of a shared decision-making approach, PROMs can be used to assess whether the goals of treatment are being met, and to optimise treatment outcomes.
References
- Maurer M, Magerl M, Betschel S, Aberer W, Ansotegui IJ, Aygören-Pürsün E, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2021 revision and update. Allergy. 2022;77(7):1961-90.
- Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: A randomized clinical trial. JAMA. 2018;320(20):2108-21.
- Longhurst H, Cicardi M, Craig T, Bork K, Grattan C, Baker J, et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. N Engl J Med. 2017;376(12):1131-40.
- Zuraw B, Lumry WR, Johnston DT, Aygören-Pürsün E, Banerji A, Bernstein JA, et al. Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: A randomized, double-blind, placebo-controlled phase 3 trial. J Allergy Clin Immunol. 2021;148(1):164-72.e9.
- Maurer M, Magerl M, Ansotegui I, Aygören-Pürsün E, Betschel S, Bork K, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2017 revision and update. Allergy. 2018;73(8):1575-96.
- SPC Takhzyro 11/8/23
- SPC Orladeyo 30/4/21
- Medicinrådet. Medicinrådets lægemiddelrekommandation vedr. lægemidler til forebyggende behandling af arveligt angioødem. 2024, Version 1.0.
- Craig T. Triggers and short-term prophylaxis in patients with hereditary angioedema. Allergy Asthma Proc. 2020;41(Suppl 1):S30-s4.
- SPC Berinert 500/1500 IU 23/12/21
- SPC Berinert 2000/3000 IU 11/5/22
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: A model for clinical practice. J Gen Intern Med. 2012;27(10):1361-7.
- Settipane RA, Bukstein DA, Riedl MA. Hereditary angioedema and shared decision making. Allergy Asthma Proc. 2020;41(Suppl 1):S55-S60.
DNK-AND-0001, 25/04/25




